Abstract
Background:
Blast phase (BP) of chronic myeloid leukemia (CML) remains a major therapeutic challenge in the era of tyrosine kinase inhibitor (TKI) therapy. Given the lack of effective treatment for BP, a potential key to improve patient outcome lies in identifying patients at high risk of progression and preventing onset of BP. Additional chromosomal abnormalities (ACAs) are important determinants of patient outcome. Despite its rarity in TKI era, BP develops in most CML patients in pre-TKI era regardless of ACAs. It is unknown how the development of BP is differently affected by ACAs in the TKI era.
Design:
We investigated the value of ACAs in prediction of BP in a large cohort of CML patients. Inclusion criteria included: 1, received TKIs as part of therapy; 2, age at diagnosis > 18 years; 3, presence of t(9;22) or variant translocations detected by conventional karyotyping. All cytogenetic abnormalities in Ph+ cells except -Y were considered ACAs. We examined the latencies from initial CML diagnosis to ACA emergence (Interval 1), from ACA emergence to BP (Interval 2) and survival after BP (Interval 3).
Results:
A total of 2,326 patients were included in the study, including 1,322 men and 1,004 women with a median age at diagnosis of 49 years (range, 18-88 years). In total, 570 had ACAs, including 333 (58.4%) with a single ACA and 237 (41.5%) with multiple ACAs. The most common single ACAs were: +8, +Ph, 3q26.2 rearrangement, i(17q), and -7/7q-, with a frequency of 13.5%, 12.6%, 7.5%, 6.6%, 3.9%, respectively. Of 237 patients with complex ACAs, 18 had 3q26.2 rearrangement, 19 had -7/7q-, and 30 had i(17q) as a component of complex ACAs. Seven patients had two of these three ACAs in different combinations. These complex ACAs were grouped together as high-risk complex ACAs. Complex ACAs without these three chromosomal abnormalities were designated as other complex ACAs.
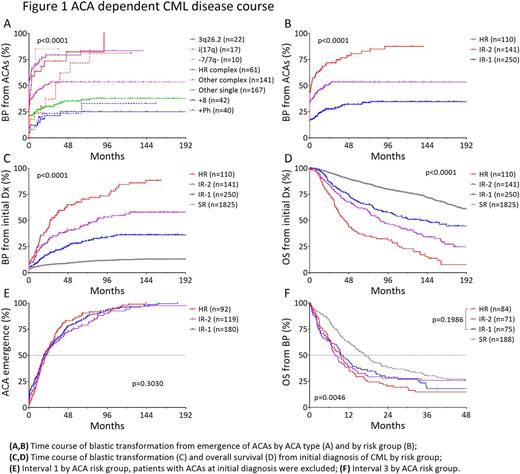
Patients with 3q26.2 rearrangement, -7/7q-, i(17q) and high-risk complex ACAs had a significantly faster blastic transformation, with a median Interval 2 of 2.8, 0, 32.6, 0 months, respectively, compared with 19.2 months in patients with other complex ACAs, and unreached in patients with +8, +Ph, or other single ACAs, and (Fig 1A). Based on the frequency and velocity of blastic transformation, patients were stratified into four risk groups. Patients without ACA formed the standard-risk (SR) group, patients with isolated 3q26.2 rearrangement, -7/7q-, or i(17q) and high-risk complex ACAs formed the high-risk (HR) group, patients with +8, +Ph or other single ACAs formed the intermediate-1 risk (IR-1) group, and patients with other complex ACAs formed the intermediate-2 risk (IR-2) group. By risk group, the median Interval 2 was unreached for IR-1group, 19.2 months for IR-2 group, and 1.9 months for HR group. (Fig 1B), and the interval from CML diagnosis to BP (Interval 1 + Interval 2) was unreached for SR and IR-1, 83.7 months for IR-2 patients, and 58.2 months for HR patients (Fig 1C). This risk of BP correlated well with patient survival from CML diagnosis (Fig 1D).
The disease course of CML patients with ACAs follows a triphasic course, that is, Interval 1, 2 and 3. There was no or little variation in Interval 1 among three ACA groups (Fig 1E). No significant difference in Interval 3 was observed among three ACA groups neither (Fig 1F). SR patients had only slightly better survival of 17.0 months, compared with 9.3 months in other 3 groups combined (Fig 1F). The lack of difference in Interval 1 and 3 indicate that the predominant determinant of CML disease course in TKI era lies in the duration of Interval 2, which is in turn highly ACA-dependent.
Conclusions:
Chromosomal alterations predict the risk of blastic transformation in CML patients treated with TKIs. Patients can be stratified into four different cytogenetic risk groups based on ACA-dependent risk of blastic transformation. This risk stratification highly correlates with patient survival. By prolonging the duration of Interval 2, TKI therapy mitigates the risk of blastic transformation associated with low-risk ACAs or no ACAs but does not alter the natural course of CML associated with high-risk ACAs: 3q26.2 rearrangement, -7/7q- and i(17q). Thus, we identify a group of patients who are at a high risk of rapid disease progression. These patients may benefit from alternative treatment approach including early allogeneic hematopoietic stem cell transplantation to prevent blastic transformation.
Cortes: ARIAD: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; Teva: Research Funding; Novartis Pharmaceuticals Corporation: Consultancy, Research Funding; Sun Pharma: Research Funding; Pfizer: Consultancy, Research Funding; BMS: Consultancy, Research Funding. Kantarjian: Bristol-Meyers Squibb: Research Funding; Amgen: Research Funding; Pfizer: Research Funding; ARIAD: Research Funding; Novartis: Research Funding; Delta-Fly Pharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal